Vaccination Exploration
How Emory got involved in developing and testing vaccines, from working on HIV-AIDS efforts decades ago to participating in phase 3 COVID-19 trials today.

In a small exam room on the Emory University campus, Dorothy Scott pushes up the short sleeve on her glittery gold top. A nurse checks her blood pressure and scribbles down the results. A few minutes later, Scott winces as a needle pierces her vein.
Scott, of Braselton, has just become the newest participant in one of the largest and most promising COVID-19 vaccine trials in nation. She is ninety-one years old.
“I wanted to help,” she says.
As the coronavirus pandemic has swept across the nation, volunteers like Sco, have stepped up as everyday heroes. Tens of thousands of them have arrived at vaccine testing sites to roll up their sleeves for science.
One of those sites is Emory, which has emerged as key player in the global race to find a vaccine. Emory was one of the first sites to enroll participants in the nation’s inaugural COVID vaccine trial back in March. It’s home to the Infectious Disease Clinical Research Consortium (IDCRC), which is helping to steer national COVID research efforts. A homegrown vaccine candidate is also in the works, fueled by a large federal grant.
The vaccines—along with other COVID-19 research and clinical trials—have trained a spotlight on Emory’s deep roots in the worlds of infectious diseases. In a matter of months, COVID research across the university has attracted nearly $90 million in outside funding.
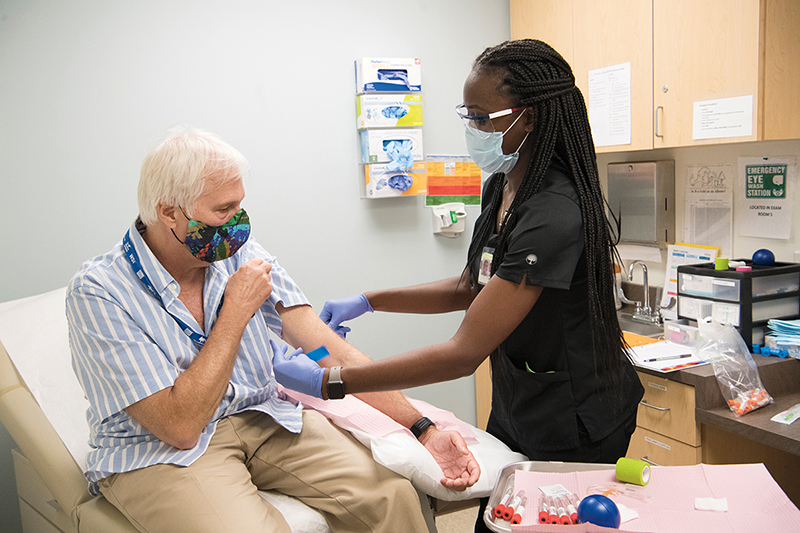
COVID-19 VACCINE TRIAL UNDERWAY About 750 volunteers are expected to enroll at three Emory sites as part of the Phase 3 trial for a vaccine candidate codeveloped by Moderna and the National Institute of Allergy and Infectious Diseases (NIAID).
The stakes couldn’t be higher. The death toll from COVID-19 continues to rise. The economy is reeling. Schools are struggling to educate kids remotely. There is widespread agreement among public health experts that a safe and effective vaccine is the single best way to return to normal.
“There has been no other time in history where we’ve needed to develop a vaccine so quickly,” Walter Orenstein, professor and associate director of the Emory Vaccine Center. “This is, by far, the most serious pandemic in our lifetime.”
The Ten Dollar Deal that Changed Atlanta
In some ways, Emory has been preparing for moments such as this since World War II. In the American South, soggy and searingly hot conditions were an ideal breeding ground for mosquitos. Troops stationed in the region were becoming infected with malaria at alarming rates. In response, the federal government established a temporary public health agency in Atlanta.
With the end of the war, officials realized the value of an organization devoted to communicable diseases. Since a critical mass of experienced disease fighters were already in Georgia, that’s where they stayed.
In 1947, Coca-Cola Company president and philanthropist Robert W. Woodruff lured the fledgling public health agency from downtown Atlanta to a plot of land adjacent to Emory’s campus. The federal government would pay a token ten dollars for fifteen acres of land on Clifton Road. The agency would go on to become the US Centers for Disease Control and Prevention (CDC). In other words, Emory and the CDC grew up together as neighbors.
“The CDC is very much a part of the Atlanta and Emory communities,” says David S. Stephens, professor and chair of the Department of Medicine in Emory University School of Medicine and vice president for research of Emory’s Woodruff Health Sciences Center. “Why Atlanta? Why Emory? I think it goes back to decisions that were made some seventy years ago, that led to the CDC being established here. That has attracted a lot of people to Atlanta for public health and vaccines.”

PHASE 3 OF THE TRIAL IS MUCH LARGER, Enrolling some thirty-thousand adult volunteers across eighty separate sites.
A New Center and a Star Recruit
Over the years, Emory’s reputation in health sciences continued to grow. But in the 1990s, an ambitious new plan took shape that would catapult the university to the next level.
In 1992, the CDC announced a grim milestone: AIDS had become the number one cause of death for men ages twenty-five to forty-four. That same year, Richard Compans arrived at Emory and took over as the chairman of the School of Medicine’s Department of Microbiology and Immunology. Compans had been researching an HIV vaccine at the University of Alabama at Birmingham before coming to Atlanta and wanted to continue to build on that work. At the same time, a new organization was just getting off the ground that would prove invaluable. The Georgia Research Alliance (GRA), jointly funded with public and private investments, was tasked with making the state a magnet for scientific innovation. A centerpiece of GRA’s strategy was an eminent scholar program designed to help academic institutions attract extraordinary talent.
“I thought vaccine development might appeal to GRA and it did,” Compans recalls. Armed with funding, Compans went in search of a vaccine eminent scholar. He knew right away who he wanted for the job.
Out in Los Angeles, Rafi Ahmed was happily ensconced in a lab at UCLA. His cutting-edge research on the fundamentals of immune memory had made waves, and he had become something of a rock star in the world of immunology. When he got a phone call about starting a new vaccine center at Emory, he knew very little about the school or Atlanta.
“It was a new concept at the time,” Ahmed says of the idea of a vaccine center. “There were maybe two or three others in the country.”
“Everyone told me I was crazy to leave UCLA for Emory,” Ahmed says. “But I just felt that there was this incredible opportunity.”
UCLA had the luster of a massive research university. But at Emory, he would have the chance to build a vaccine research center from the ground up. The CDC was part of the attraction. So was the Yerkes National Primate Research Center, a world-class facility vital to the kind of research Ahmed wanted to do.
He arrived in Atlanta in 1995 and never looked back. “A lot of wonderful things happened. It all took off so quickly,” says Ahmed, who serves as the Emory Vaccine Center’s top director.
The center opened its twenty-thousand square feet of office and lab space on the campus of the Yerkes facility. Each floor has a Biosafety Level 3 lab, able to safely handle some of the most dangerous viruses on the planet. The center pulls infectious disease and pediatric specialists from across Emory, including from the School of Medicine and Rollins School of Public Health. The Hope Clinic was soon created as the Vaccine Center's clinical arm, conducting human trials to test the safety efficacy of vaccines and other drugs.
Orenstein, a veteran of the Bill and Melinda Gates Foundation and former director of the United States Immunization program at the CDC, came aboard to lend vaccine policy expertise.
The Emory Vaccine Center was now not just about basic science, but about clinical trials, translational studies, and public policy. “It’s a very unique set up,” Ahmed says. “Soup to nuts”
COVID-19 Vaccine Trials
The center’s signature work on HIV has proven useful in the pandemic. In May, Rama Amara, an HIV researcher at the Vaccine Center and Yerkes, received a $582,000 two-year grant from the National Institute of Allergy and Infectious Diseases (NIAID) to transform his research on an HIV vaccine into one that could work on the coronavirus.
The first vaccine candidate to make it to human trials in the United States was one codeveloped by pharmaceutical company Moderna and NIAID. Emory was one of just three sites to enroll participants for Phase 1.
Early results are encouraging. In a pair of papers published in the New England Journal of Medicine, researchers said that in Phase 1, mRNA-1273 was generally well tolerated and produced a strong immune response in healthy adult volunteers, both young and old.
The lead author on one of those papers is Evan Anderson, a professor at Emory’s School of Medicine and a principal investigator for the vaccine trial at Emory-Children’s Center. His paper showed that the vaccine stimulated a strong immune response in participants fifty-six years and older. Anderson said that’s a hopeful sign because immune response generally declines with age.
Now a much larger Phase 3 trial is underway. Some thirty-thousand volunteers will participate at eighty sites around the country. Emory is expected to enroll about 750 people at the Hope Clinic, Emory-Children’s Center, and the Ponce de Leon Center for Clinical Research. Moderna says that if everything continues to go well in the trial, the company could apply to federal regulators for emergency use authorization before the end of the year.
Emory is expected to host other vaccine trials as well later in the year. “Without a vaccine, I don’t think that we can really move past COVID,” said Anderson, who is also a pediatrician at Children’s Healthcare of Atlanta.
Phase 3 of the vaccine trial is randomized and placebo controlled, meaning participants—like Dorothy Scott—don’t know if they are being injected with the vaccine or saline. Volunteers are watched closely for two years to see if they contract COVID-19 as they go about their daily lives.
While the trial is enrolling older adults—who are at higher risk of having serious complications from COVID—Scott remembers the race for a vaccine to combat another lethal menace: polio. The retired insurance office worker says those days were scary; the polio virus left some paralyzed and others dead.
Scott was in her mid-twenties when a vaccine emerged to combat the disabling disease, and she hasn’t thought much about it since then. Until now. “This,” Scott says, referring to the COVID-19 pandemic, “is even worse.”
COVID-19 Vaccine Candidate Uses Groundbreaking MRNA Technology
The COVID-19 vaccine candidate that researchers are testing on hundreds of volunteers at Emory University could make history. If it’s proven safe and effective, it might become the first commercially available vaccine to use mRNA technology to prevent infection in humans.
To understand what that means, it’s important to understand how vaccines have traditionally worked. To build immunity, doctors typically inject patients with small amounts of the very germ they are trying to protect against. Sometimes it’s a live, but weakened version; that’s the case with the measles, mumps, and rubella (MMR) vaccine. Other times the vaccine is inactive, meaning scientists have killed the virus or bacteria. Polio and rabies are examples.
But with mRNA technology, the patient is never injected with a virus, either living or dead. Instead, scientists are trying to trick the body’s immune system by essentially hacking socalled messenger RNA.
Scientists using this technique create a synthetic mRNA strand that carries the genetic code for the part of the virus. That’s packaged into nanoparticles and shuttled into cells swaddled in a protective layer of lipids. When the body reads the code it thinks a virus is present and springs into action, manufacturing antibodies to fight the invader.
The mRNA approach isn’t brand new. Researchers have been tinkering with the idea for decades. But in recent years, new scientific advances and funding have breathed fresh life into the field. The urgency of a global pandemic has kicked the effort into overdrive.
Of the leading candidates in the federal government’s “Operation Warp Speed” COVID-19 vaccine program, two use mRNA platforms.
Emory is participating in the clinical trial of mRNA-1273, an investigational vaccine codeveloped by Moderna, a Cambridge, Massachusetts-based biotech company, and the National Institute of Allergy and Infectious Diseases (NIAID). Pfizer/bioNTech also has an mRNA vaccine in clinical trials.
Rafi Ahmed, director of the Emory Vaccine Center, called the mRNA technology “promising.” But he also struck a note of caution. “There is no proven record of how it works as a licensed vaccine,” Ahmed says.
Still, Ahmed believes that the technology is appealing as a means to combat the growing COVID-19 pandemic for one key reason: speed.
Traditional virus-based vaccines can take years to refine, grow, and scale up for mass production. A synthetic mRNA vaccine could be created in a lab in a matter of days, allowing massive amounts to be manufactured rapidly.
“It’s very quick,” Ahmed says. “And it should give good antibody response.”
The speed of COVID-19 vaccine development has been helped along by the nature of the virus. Researchers had already been working to counter the SARS virus, which fueled a deadly outbreak in 2003. That research had identified the unique spike protein as a promising vaccine target.
Some of that work has been repurposed into development of a COVID-19 vaccine. Labs are moving at a rapid pace, building on the rich repository of research already in hand.
But labs are one thing. The true test for mRNA-1273 will come in the months-long Phase 3 clinical trials that ask one simple question: Does it work?





